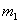 is the mass of kinetic
fuel from outer space impacting on the aerobrake in a small time
interval,
is the mass of kinetic
fuel from outer space impacting on the aerobrake in a small time
interval, 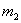 is the mass
of the on-board fuel which is added at the same time, and
is the mass
of the on-board fuel which is added at the same time, and 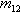 is the combined mass that
expands backwards into space, etc.
is the combined mass that
expands backwards into space, etc.How much more impulse do we get from inverted aerobraking if we add more on-board fuel? This on-board fuel can be used to cool down the aerobrake. At first I will assume that this extra fuel does not carry any chemical energy, nor does it consume any chemical energy when mixed with kinetic fuel. All energy comes from the kinetic fuel. This energy becomes thermal energy on impact on the aerobrake. I.e. the fuel turns into extremely hot gas, maybe even into plasma. Adding on-board fuel at this point will cool down the gas/plasma a bit and heat up the on-board fuel. More gaseous mass is expanding backwards into space, but at a lower speed. Still, as we will see, overall more thrust will be generated. But bringing on-board fuel from earth is expensive. How much more thrust do we get?
More specifically, we are interested in the impulse the space ship gets when on-board fuel is used. Let be:
I: impulse
v: velocity
m: mass
N: number of
particles in the gas
n: mass per
particle
T:
termperature in degree Kelvin
R: gas constant
L: Avogardros
constant
Index 1 refers to the fuel from outer space, 2 refers to the
on-board fuel. Index 12 means both combined. E.g. 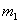 is the mass of kinetic
fuel from outer space impacting on the aerobrake in a small time
interval,
is the mass of kinetic
fuel from outer space impacting on the aerobrake in a small time
interval, 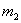 is the mass
of the on-board fuel which is added at the same time, and
is the mass
of the on-board fuel which is added at the same time, and 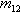 is the combined mass that
expands backwards into space, etc.
is the combined mass that
expands backwards into space, etc.
Total impulse is the sum of impulse of impact of the kinetic fuel and impulse of the expanding mix of kinetic fuel and on-board fuel.
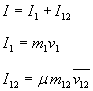
where µ is the degree of efficiency of the aerobrake to collect this impulse (µ is positive and less or equal to 1).
Energy of the kinetic fuel is
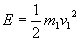
which is converted into thermal energy on impact. Average energy of a particle in the mixed fuel is
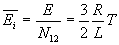
From this we can compute temperature of the fuel mix as
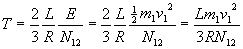
The average squared velocity of particles in the mix is
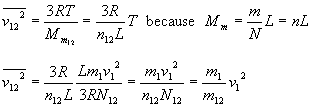
So average squared speed of the expanding gas does not depend on what kind of on-board fuel we use, as long as it is a gas. (If it is a fluid that needs some energy to be turned into a gas, or if it reacts with the kinetic fuel chemically and adds some energy, we have to consider that. See below. But it is not important what weight a resulting gas particle has.)
For impulse we need average velocity. Squared average velocity in a gas is not the same as average squared velocity. Assuming speed distribution in the gas stays the same while the gas is expanded, the relation is
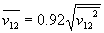
Putting this together using
ß = 0.92 µ
as the "bounce factor" we get a total impulse of
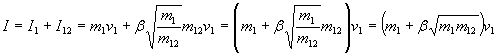
So basically the important thing is the relation of kinetic fuel to
on-board fuel. Defining the fraction 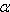 of on-board fuel as
of on-board fuel as
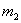 =
= 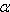
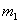
we get (see figure 1):
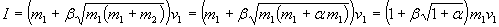
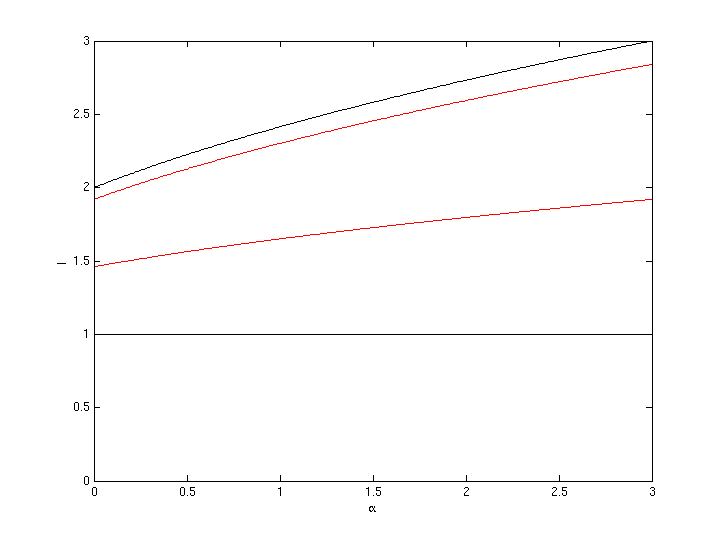
Figure 1: total impulse (related to the speed difference v1 between ship and kinetic fuel and mass of kinetic fuel m1 used) at a given fraction ß of on-board fuel (related to the kinetic fuels mass) for different bounce factors ß (black lower curve: ß=0, black upper ß=1, red lower ß=0.46, red upper ß=0.92).
The more added fuel, the more impulse. The more bounce, the more impulse too. Quite as one would expect.
If we don't add on-board fuel, i.e. 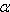 = 0, impulse is
= 0, impulse is 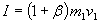 , so ß
is in deed the same bounce factor that is used in ImpulseTransfer (at least if ships mass
is much larger than fuel mass "per bounce").
, so ß
is in deed the same bounce factor that is used in ImpulseTransfer (at least if ships mass
is much larger than fuel mass "per bounce").
Finally we can relate impulse to the total amount of fuel used (figure 2):
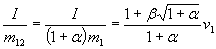
Example:
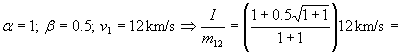
= 0.85·12 km/s =10.2 km/s; specific impulse of fuel is 1040
seconds; 2.3 times better than chemical fuel.
Second Example:
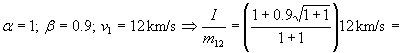
= 1.1·12 km/s = 13.6 km/s; specific impulse of fuel is 1390
seconds; 3 times better than chemical fuel.
Third Example:
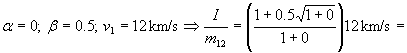
= 1.5·12 km/s = 18 km/s; specific impulse of fuel is 1830
seconds; 4 times better than chemical fuel.
Fourth Example:
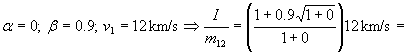
= 1.9·12 km/s = 22.8 km/s; specific impulse of fuel is 2320
seconds; 5 times better than chemical fuel.
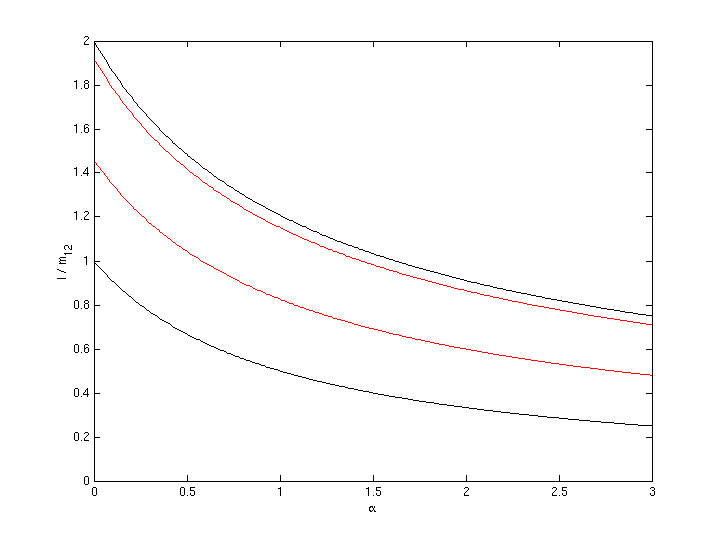
Figure 2: overall impulse factor per fuel mass unit
if on-board fuel is added at an amount of 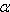 times the kinetic fuel. Curves vary with bounce factor
ß (look
left and subtract 1 to get ß). Red curves
are for minimal and maximal estimated expected ß (see ImpulseTransfer).
times the kinetic fuel. Curves vary with bounce factor
ß (look
left and subtract 1 to get ß). Red curves
are for minimal and maximal estimated expected ß (see ImpulseTransfer).
If velocity of kinetic fuel is 12 km/s, conventional chemical fuel has an impulse per mass of about 0.4 in figure 2. So adding a considerable amount of on-board fuel still gives better performance than todays chemical rockets.
But the main result is: Overall impulse per kilogram of total fuel gets lower the higher the fraction of on-board fuel becomes!
Adding on-board fuel is only a good idea
a) if we have to cool the aerobrake, or
b) if we want to compensate for temporary kinetic fuel density changes
to
- keep the plasma shield alive, or
- protect
heat shield against ablation, or
- and get a less "bumpy" ride, or
c) if we use it to stabilize the ship
(by controling which side of the aerobrake gets how much more on-board
fuel), or
d) if bringing kinetic fuel is more expensive than bringing on-board
fuel
The last point may sound like a contradiction, because inverted aerobraking assumed cheap fuel from space in the beginning. But note that specific impulse for combined kinetic and on-board fuel can be much higher than specific impulse for chemical fuel. So inverted aerobraking could even be economic if bringing fuel from outer space is more expensive than bringing it from earth. Costs of bringing on-board fuel vary with phase of launch, too.
(Todo: check these calculations again; they look too good.)
Example: oxygen as kinetic fuel, hydrogen as on-board fuel.
We need to know: how much energy added. Known about rocket fuel is
exhaust velocity or specific impulse.
Energy is proportional to used on-board fuel:
E = f·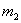
Let c be ratio of mass of on-board fuel to mass of kinetic fuel that reacts with each other.
Example: 2·H2+O2=2·H20 gives
c = (2·2·1u)/(2·16u) = 4/32 = 1/8 = 0.125,
since hydrogen has atomic mass 1 and oxygen has atomic mass 16.
c = 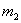 /
/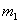 ',
',
where 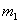 ' <=
' <= 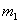 , or any part of the
on-board fuel above that threshold will not be burnt and must be
counted as non-chemical on-board fuel. This means:
, or any part of the
on-board fuel above that threshold will not be burnt and must be
counted as non-chemical on-board fuel. This means:
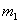 >=
>= 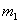 ' =
' = 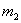 /c =
/c = 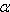 ·
·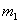 /c
/c
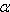 /c <= 1
/c <= 1
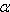 <= c
<= c
Example: a <= 0.125
Energy to heat up 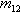 is
is
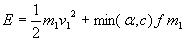
Similar to the above we get:
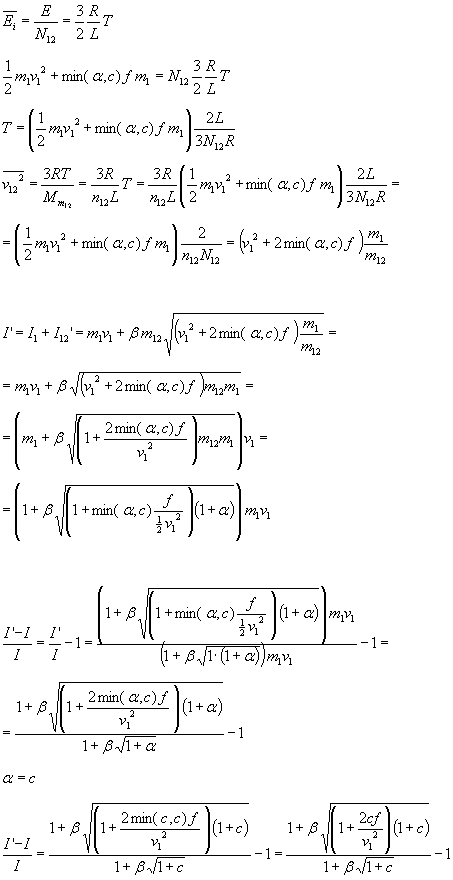
If kinetic fuel is oxygen, f is the lower heating value (LHV) of the on-board fuel. Hydrogen has a LHV of 120 MJ/kg. This gives the following figure:
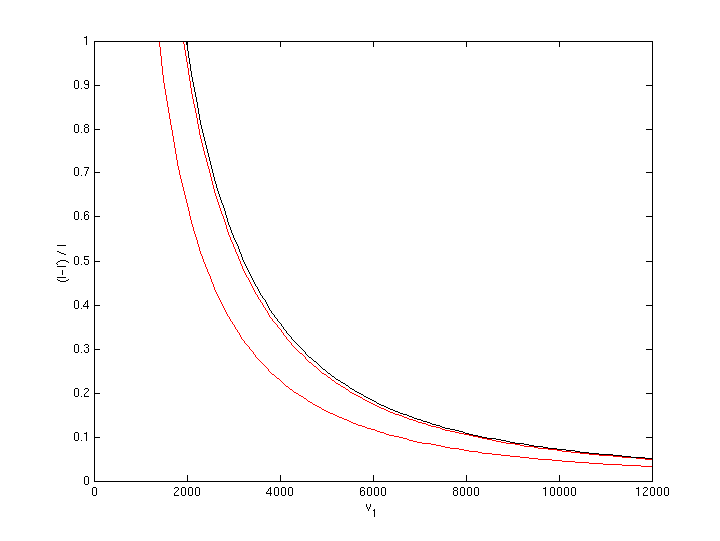
So during most of the inverted aerobraking maneuver additional impulse from chemical energy is very low compared impulse from kinetical energy, e.g. 5% to 15%. Only during the last phase chemical energy gives a significant contribution. But maybe we should better use a traditional rocket engine for that part?
Let's assume we would bring the same amount of fuel as the on-board fuel and burn it in a rocket engine. This means, we also have to bring oxidizer, which is to be included in the total amount to get a fair comparison. Assuming an ideal rocket engine that converts all the chemical energy into impulse using all of the fuels mass, we get an alternate impulse value, which we can be related to the added impulse from the chemical fuel when used as on-board fuel for inverted aerobraking:
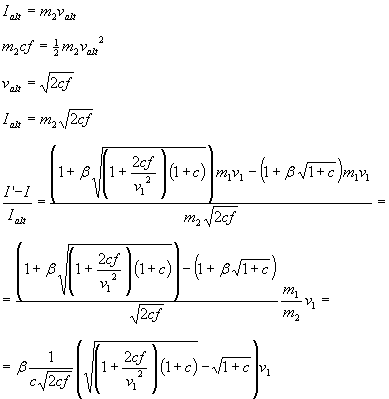
Plotting this formula gives:
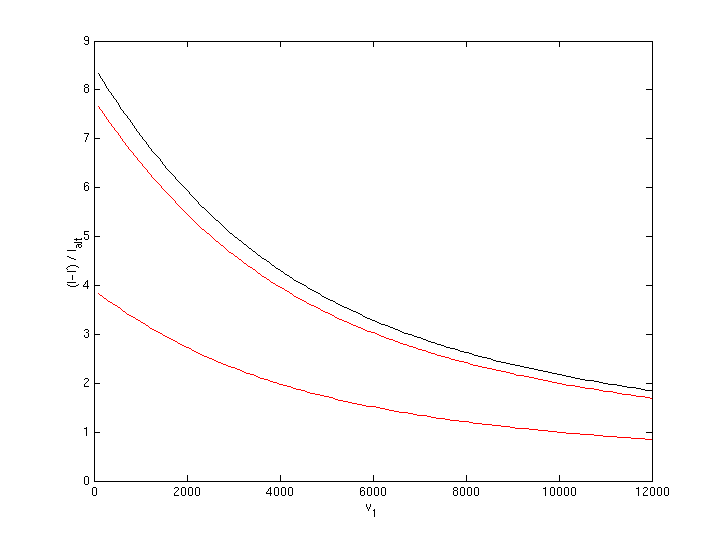
Remember these curves result if hydrogen is used as on-board fuel, oxygen is delivered via kinetic fuel and all of combined fuels turns into water vapor. At high velocities, the impulse achieved is up to almost twice as much as what an ideal rocket engine could provide. When velocity of kinetic fuel becomes low (which is when inverted aerobraking becomes inefficient and could use some boost), the gain is 4 to 8 times!
So adding fuel which carries chemical energy is an attractive
option, especially for low ![]() v
or if kinetic fuel in LEO is cheaper than fuel brought from earth to
orbital height.
v
or if kinetic fuel in LEO is cheaper than fuel brought from earth to
orbital height.
But note that the above calculations assume perfect combustion on
top of the aerobrake. This may not be true. Furthermore combustion will
probably behave quite different for very low ![]() v values than for high
v values than for high ![]() v values. More detailed calculations should be
done on that.
v values. More detailed calculations should be
done on that.
Critical remarks: All calculations above assume fuel mass "per bounce" to be small compared to ships mass (pretty realistic, I'd say). Calculations above are for "ideal" gas. Is there a big difference for a "real" gas, especially if it is a mix of different gases, e.g. water and hydrogen? (I don't expect much difference, but ...) Does plasma behave like an ideal gas? (Again I would say it does, but ...) Finally the process of mixing kinetic and on-board fuel might be slow compared to the "turn around" time of kinetic fuel. This might reduce efficiency for on-board fuel a bit. (Once more I'd say not too much ...)